Not all medical waste is created equal, and therefore, not all of it necessitates or is suitable for incineration. However, for certain categories of medical bio-waste, incineration is not only the preferred method but often a regulatory requirement due to the high risks these wastes pose to public health and the environment. The decision to incinerate specific waste streams is typically guided by their potential to cause infection, their chemical hazards, or their physical characteristics that make other treatment methods less effective or unsafe. Proper waste segregation at the point of generation is paramount to ensure that only waste requiring incineration is directed to this process, optimizing efficiency and minimizing costs and environmental impact. Understanding which types of medical waste demand incineration is crucial for compliant and responsible healthcare waste management.
One of the primary categories of waste that frequently requires incineration is pathological waste. This includes human or animal tissues, organs, body parts, and surgical specimens. Due to their organic nature and potential to harbor infectious agents, as well as aesthetic and ethical considerations, incineration is often mandated for pathological waste to ensure complete destruction and sterilization. Similarly, waste contaminated with prions, the infectious agents responsible for diseases like Creutzfeldt-Jakob disease, requires the high temperatures achieved during incineration to ensure their inactivation, as prions are notoriously resistant to conventional sterilization methods. Effective infectious waste disposal for these materials is critical.
Trace chemotherapy waste, which includes items like empty drug vials, syringes, IV bags, and contaminated personal protective equipment (PPE) that have come into contact with chemotherapeutic agents, is another significant stream often designated for incineration. While bulk chemotherapy waste (unused or partially used drugs) is typically managed as hazardous chemical waste, trace amounts can still pose risks if not properly destroyed. Incineration effectively breaks down these cytotoxic and genotoxic compounds, preventing their release into the environment. The high temperatures ensure the chemical bonds of these potent drugs are broken, rendering them inert. This is a key aspect of managing chemotherapy waste safely.
Non-hazardous pharmaceutical waste, particularly expired or unused medications that are not classified as RCRA hazardous waste, may also be directed to incineration in many jurisdictions. While some non-hazardous pharmaceuticals can be disposed of through other means, incineration offers a secure method to prevent their diversion or accidental ingestion and to destroy active pharmaceutical ingredients (APIs) that could otherwise contaminate water systems if landfilled. The decision often depends on local regulations and best management practices for pharmaceutical waste disposal.
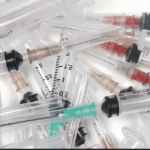
Furthermore, certain types of infectious waste, especially cultures and stocks of infectious agents from microbiology laboratories, and items heavily contaminated with blood or other potentially infectious materials, are prime candidates for incineration. While autoclaving can sterilize many infectious wastes, incineration provides an added level of security by completely destroying the waste material itself. Sharps, such as needles, scalpels, and broken glass, even after disinfection, are often incinerated to reduce their volume and render them unrecognizable and safe from causing physical injury or potential reuse. The complete destruction offered by incineration makes it a preferred method for sharps disposal in many healthcare settings. The importance of meticulous waste segregation cannot be overstated; it ensures that materials like recyclable plastics or general waste are not unnecessarily incinerated, and conversely, that all high-risk medical waste requiring incineration is properly channeled for this critical treatment process.

What Types of Medical Waste Require Incineration?
Posted in Blog.